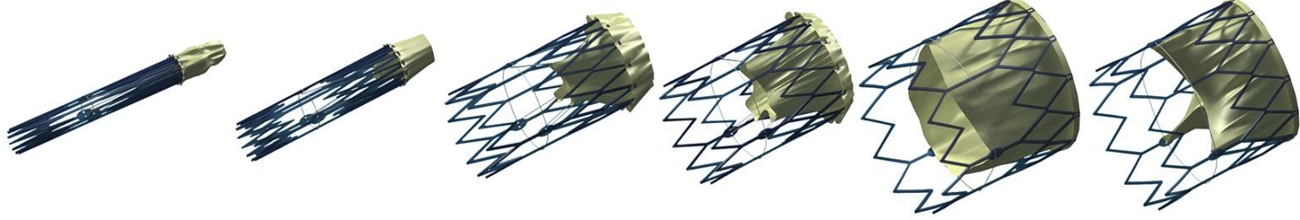
FoldaValve
Ingenuity in actionThis video shows the delivery and implantation of FoldaValve in an ovine model.
For more videos, please visit: https://goo.gl/kky9Bf
| FoldaValve is implanted with a unique delivery catheter (Ingenuity) that allows repositioning in six degrees-of-freedom when the valve is fully formed, minimizing leaks due to malpositioning. Ingenuity also allows retrieval if the implantation is unsuccessful.
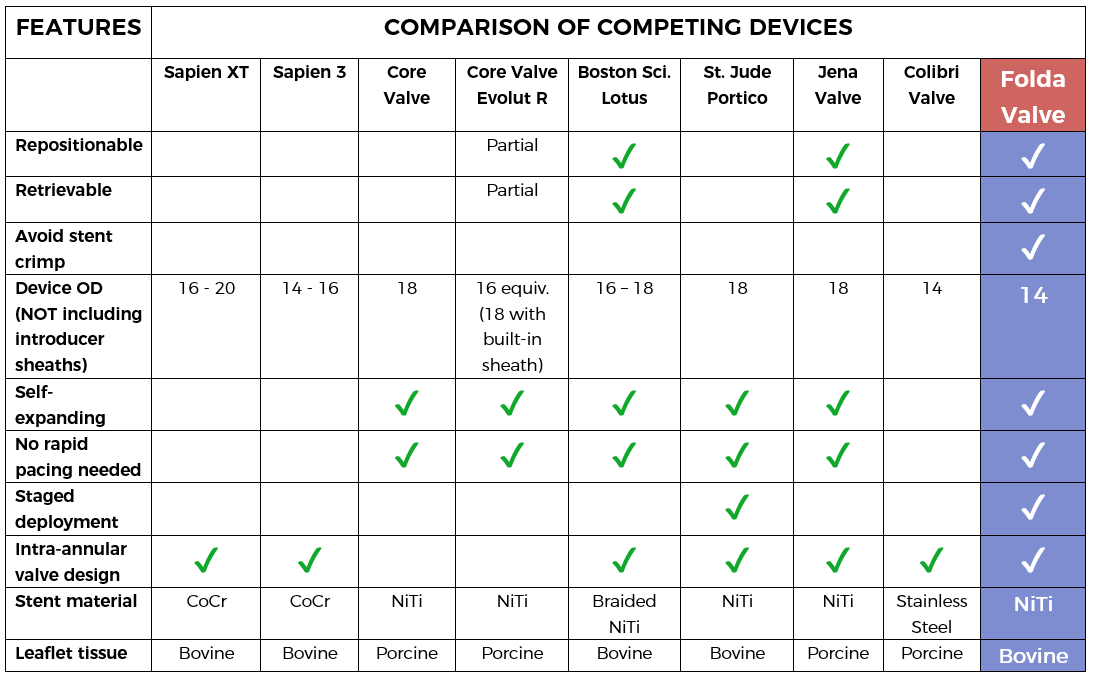
FoldaValve™ uses a unique patented draw-string mechanism to deploy the valve in situ. Via this mechanism, the leaflets are spared from being stent-crimped during delivery, preserving their quality to an extent comparable to surgical valves. FoldaValve™ has the smallest profile (14Fr OD) when compared to existing TAVRs. FoldaValve’s intra-annular design minimizes the chance of conduction abnormalities, and has an outer skirt to prevent paravalvular leakage. |
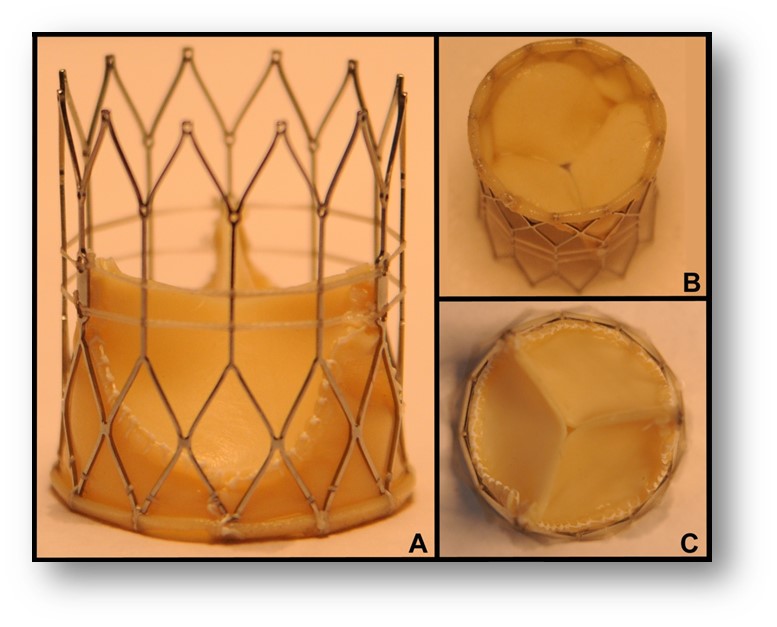
Side, top, and isometric views of FoldaValve. |
|
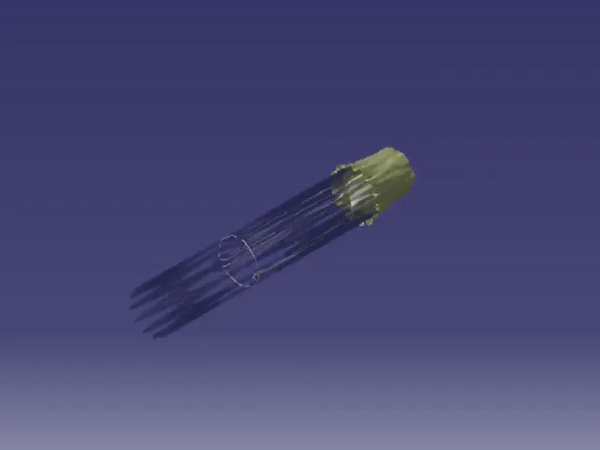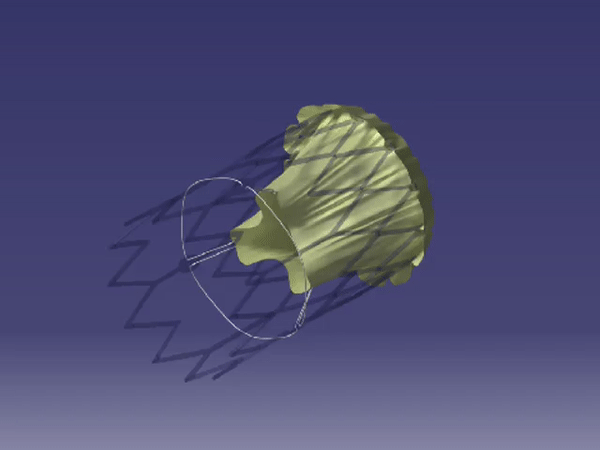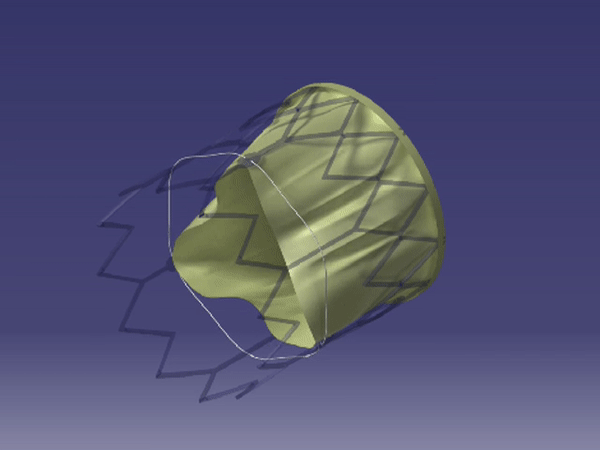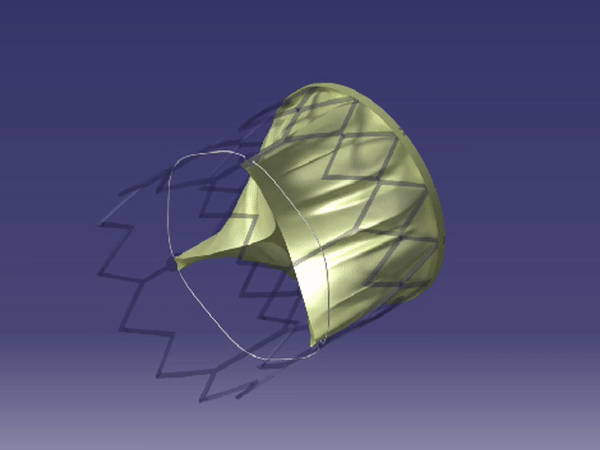
Formation of FoldaValve. |
IP PROTECTION
Patents #7,331,991, 7,780,724, 8,133,270, 8,702,788 and 8,348,999 describe FoldaValve™, and patents #9,744,037 and 9,668,859 describe FoldaValve’s delivery system, Ingenuity™.