Research Projects
Mitochondrial Transplantation
Like the nuclear genome, mitochondrial DNA is constantly prone to damage and mutations. However, mitochondria lack effective DNA repair mechanisms, so the mitochondrial DNA defects often clonally accumulate in subsequent mitochondria. The majority of mitochondrial diseases occur as a result of mutations in either nuclear DNA or mitochondrial DNA. Regardless, the phenotypic representations of all mitochondrial disorders are deficiencies in energy metabolism and cell function with cardiac involvement as a common manifestation, ranging from cardiomyopathy to arrhythmias and heart failure. While mitochondrial disease was previously considered a rare disease, collectively mitochondrial disease is relatively common, with disease causing mutations occurring in at least 1 in 4,300 Americans. What exacerbates the situation is the severity and debilitating nature of the clinical manifestation due to the vitality of the mitochondria in well-being of our cells and the fact that there is
no definitive treatment for these disorders. Additionally, mitochondrial oxidative damage/dysfunction plays a key role in complex diseases such as cardiovascular, neurodegenerative, metabolic disease, cancer, and aging.
Our goal is to investigate the intracellular consequences of mitochondrial transplantation to delineate the safety and efficacy of mitochondrial transplantation which may hold a translational potential to serve as a cellular biotherapy for patients suffering from mitochondrial damage/dysfunction.
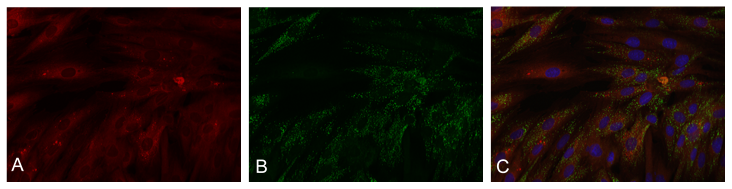
Autologous Transplantation of Mitochondria (cardiomyocytes from H9c2 cell line). (A) Transplanted Mitochondria labeled with pHrodo Red Succinimidyl Ester (B) Native Mitochondrial labeled with MitoTracker Green FM (C) Overlay, nucleus labeled with NucBlue Live.
Inspired by the endosymbiosis origin of mitochondria, we hypothesized and successfully demonstrated the feasibility of mitochondrial transplantation. Imaging was central to our research efforts and we characterized the feasibility of autologous, non-autologous, and interspecies mitochondrial transplantation through using various imaging modalities such as time-lapse microscopy, confocal imaging, holotomography imaging, structured illumination microscopy (SIM), spinning disk super resolution microscopy by optical pixel reassignment (SoRa) and flow cytometry.
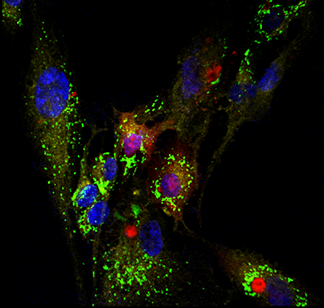
In our bioenergetics studies, we have shown that non-autologous transplantation of mitochondria (from skeletal cells into cardiomyocytes) “supercharges” the cells and leads to acutely enhanced cellular bioenergetics. At 2-day post transplantation we observed a statistically significant increase in ATP production and basal respiration in the transplant. While the bioenergetic enhancements were short-lived and the advantages diminished in long term (7, 14, and 28 days) and returned to physiological levels, there was also no apparent adverse effect. Transplant did as well as the control group in long term and there was no significant change in the production of mitochondrial superoxide production (a by-product of oxidative phosphorylation) which would be detrimental to the cell.

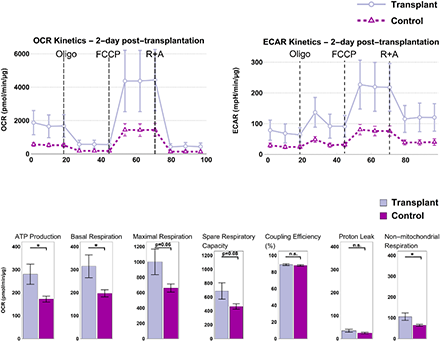
For more information please see “Bioenergetic Consequences of Mitochondrial Transplantation into Cardiomyocytes”. View all research projects
