Research Projects
Hybrid Tissue-Engineered Heart Valve
Heart valve disease remains to be a significant health problem worldwide, and consists of any dysfunction or disease process in one or more of the heart.s four valves. Currently, only two main types of prosthetic heart valves (i.e., mechanical and bioprosthetic) are available to replace a diseased valve; each has major advantages and limitations, and surgeons/cardiologists try to match the valve choice based on the patient.s particular clinical condition. Mechanical heart valves tend to last longer than bioprosthetic valves due to their stronger composition, but they carry a greater long-term risk for thromboembolism that may lead to stroke and arterial thrombosis. In contrast, bioprosthetic valves (including transcatheter valves) are expected to have a shorter lifespan but possess a biocompatible surface and improved blood flow dynamics, which minimize red blood cell damage. Each year over 110,000 heart valve replacement procedures are being performed in the U.S. while more than 80% of those are bioprosthetic, including both surgical and transcatheter.
Tissue engineering aims to resolve these issues by creating native-like structures through in vitro or in situ fabrication methods. However, purely tissue-engineered valves are unable to adjust to the dynamic loads and higher pressures in the ventricles. Thicker and/or stiffer tissues and, in some cases, leaflet retraction have been observed following implantation of these valves.
To overcome these limitations, we have developed and been testing the first patient-specific hybrid tissue-engineered heart valves with self-regenerating capacity, and potential for lifelong durability, using non-degradable scaffolds.
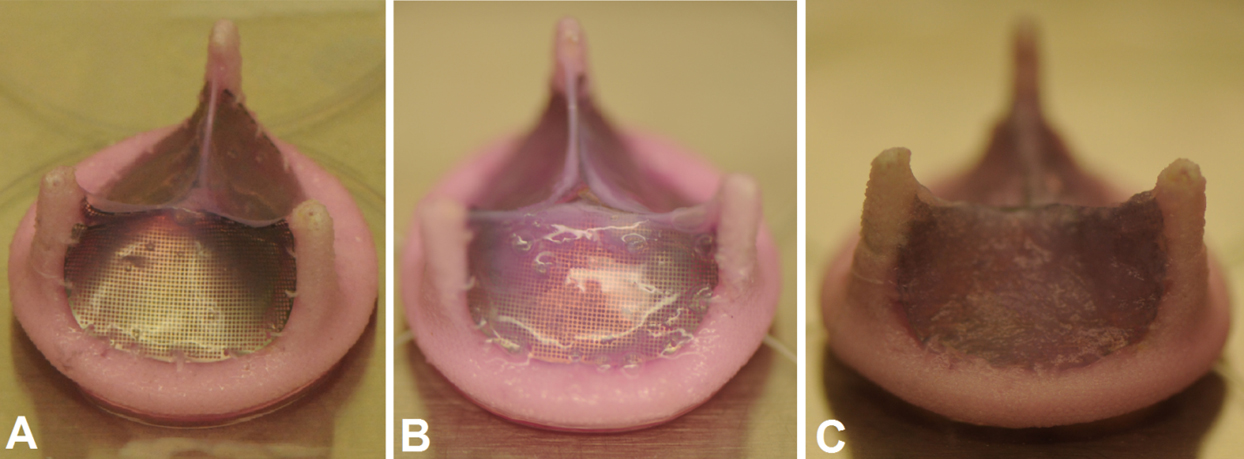
Our hybrid tissue engineered heart valve at various stages of development. Non-degradable scaffold along with (A) smooth muscle, (B) fibroblast/myofibroblast, and (C) endothelial cells, all encapsulated in collagen as the first, second, and third layers, respectively. More information can be found here.
Non-degradable scaffold - The hybrid valves. leaflets are made of flexible extra thin non-degradable scaffold tightly enclosed by tissue layers composed of patient.s own cells. The thin non-degradable scaffolds serve as the hybrid leaflet.s primary load-bearing component, preserves the engineered valve.s structural integrity when subjected to high pressure in the heart and are expected to increase durability.
We have been using a variety of materials as the scaffold. Currently, some valve scaffolds were successfully passed 50 million cycle durability test without any failure.
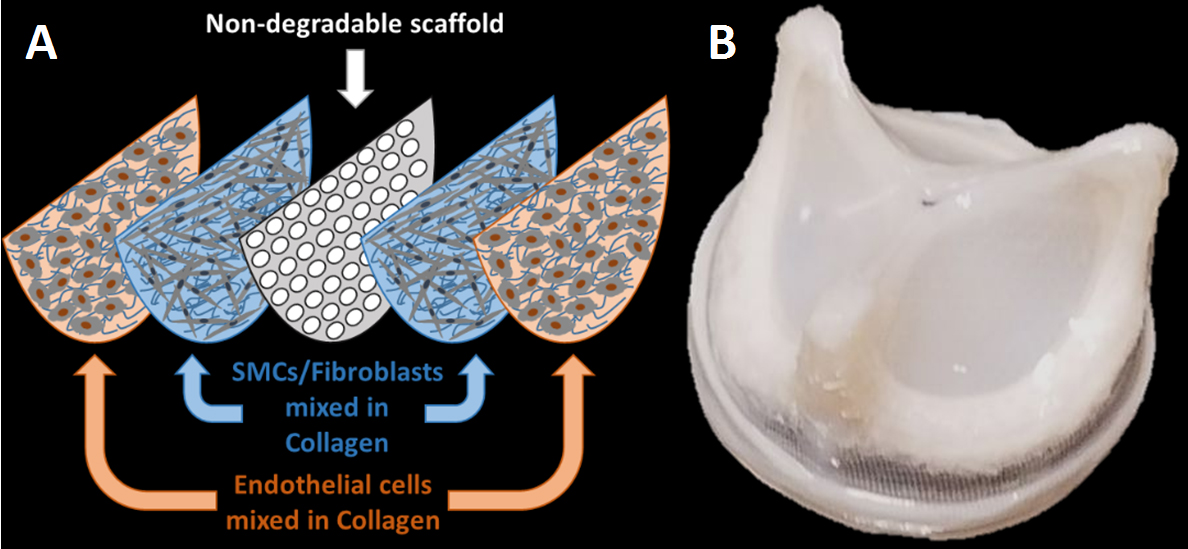
Hybrid tissue engineered heart valve used a non-degradable scaffold. (A) A schematic of non-degradable scaffold coated with the sorted patient cells in collagen, (B) the final hybrid tissue engineered valve before implantation.
Tissue Engineering - A piece of jugular or saphenous vein is needed to be surgically harvested to acquire patients. cells. Those cells will be expanded in cell-culture hood and sorted based on Magnetic-Activated Cell Sorting (MACS). The tri-leaflet hybrid valves are made by casting collagen solution mixed with a desired ratio of SMC/fibroblast layer (first layer) covered by the endothelial cells (second layer) over non-degradable scaffold. The final hybrid valve is grown in an incubator prior to valve implantation.
Tissue characterization - Preliminary Immunohistochemistry (IHC) staining has been performed on cross-sections of hybrid valve leaflet. IHC staining clearly confirms the presence of the cells enclosing the scaffold. To better understand the hybrid valve.s ExtraCellular Matrix (ECM), the percentage of each cell type on the scaffold was measured prior to
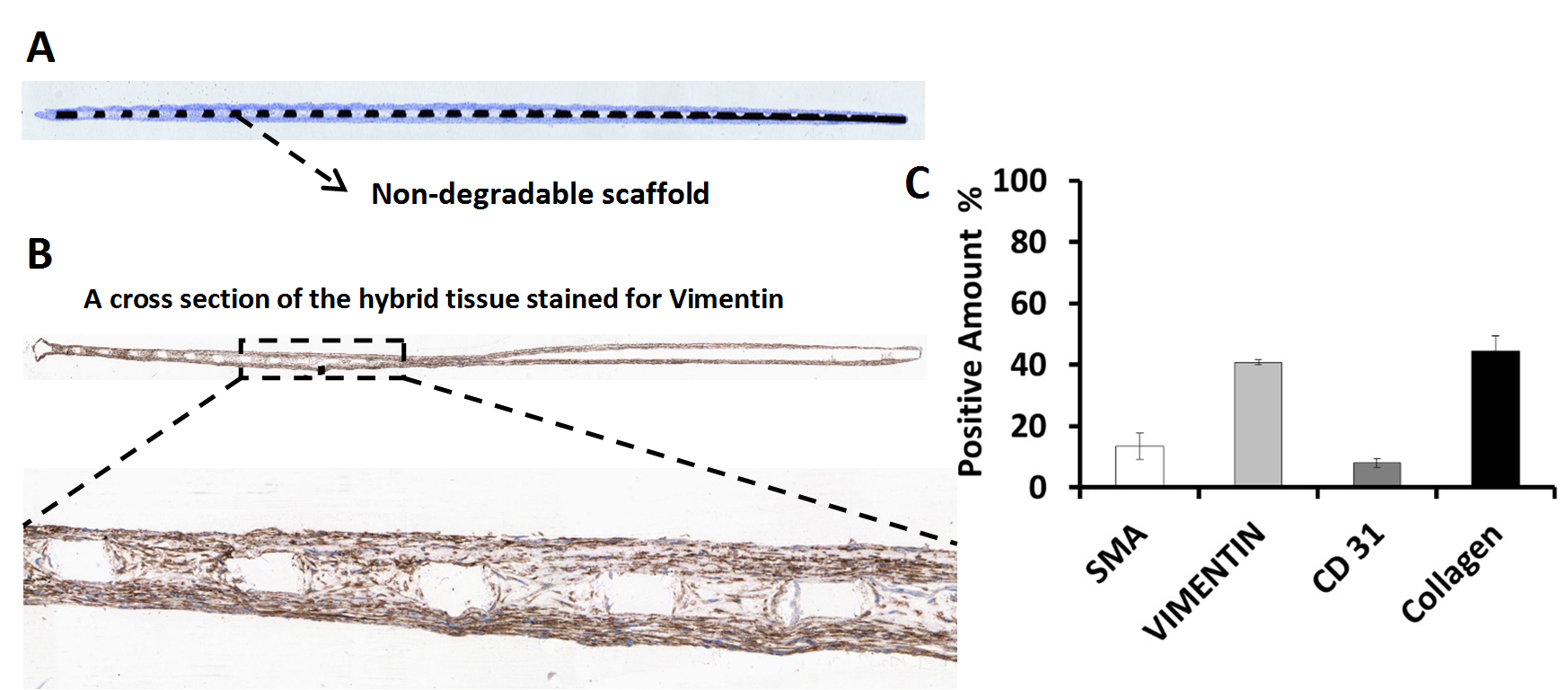
Immunohistochemisty of hybrid tissue engineered heart valve used a non-degradable scaffold. (A) A cross section of non-degradable scaffold coated with the cells in collagen (T-Blue-stained). The cell stained in blue detected on top and bottom of the non-degradable scaffold. (B) A cross section of hybrid tissue engineered heart valve stained for Vimentin. The brown zones detected with positive stain of Vimentin. (C) Quantitative percentage of cells (positive staining for SMA, Vimentin, CD31 and Collagen) in the cross section of hybrid tissue engineered heart valve.
Hybrid valve implantation - The hybrid valve has been implanted in sheep's mitral position using heart-lung bypass machine under general anesthesia at UC Irvine Medical Center. The sheep was recovered from anesthesia after the completion of the open-heart surgery. Post-procedure echocardiography showed competent valve without leakage or regurgitation.
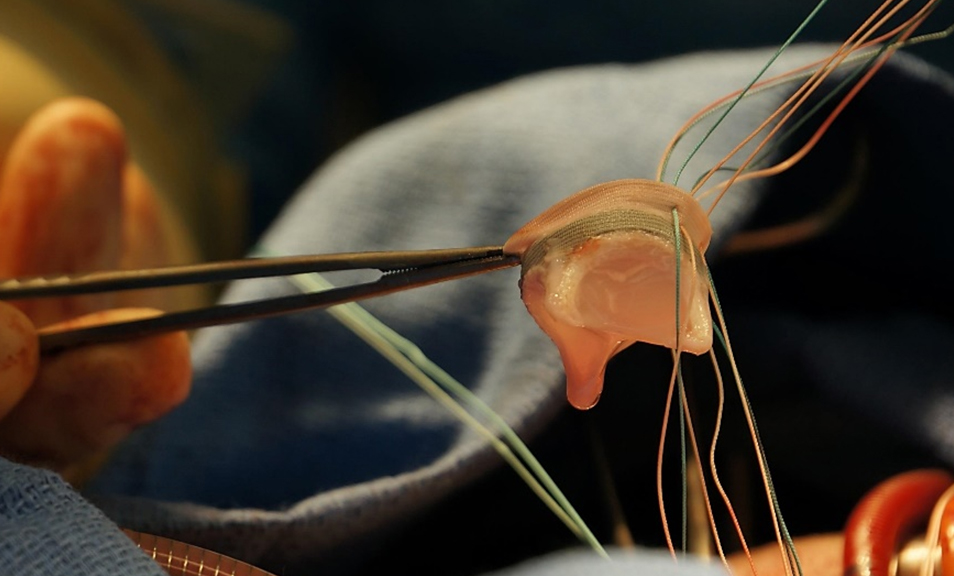
The hybrid valve was being implanted in at a sheep's mitral position via open heart surgery at UC Irvine Medical Center.
The hybrid valve promises the long-term durability of mechanical valves with the improved biocompatibility and hemodynamics of bioprosthetic heart valves.
View all research projects